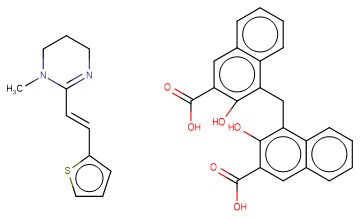
Pyrantel pamoate
CAS No. 22204-24-6
Pyrantel pamoate( Pyrantel pamoate )
Catalog No. M13543 CAS No. 22204-24-6
Pyrantel pamoate is a deworming agent in the treatment of hookworms (all species) and roundworms in domesticated animal.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 50MG | 32 | In Stock |


|
| 100MG | 45 | In Stock |


|
| 200MG | 52 | In Stock |


|
| 500MG | 67 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NamePyrantel pamoate
-
NoteResearch use only, not for human use.
-
Brief DescriptionPyrantel pamoate is a deworming agent in the treatment of hookworms (all species) and roundworms in domesticated animal.
-
DescriptionPyrantel pamoate is a deworming agent in the treatment of hookworms (all species) and roundworms in domesticated animal; acts as a depolarizing neuromuscular blocking agent.(In Vitro):Pyrantel pamoate (10 nM-10 μM; 72 h) shows good anti-A. suum and (0-168.2 M; 72 h) anti-N. americanus activity.(In Vivo):Pyrantel pamoate (10 mg/kg; p.o.; single) reduces the worms in A. ceylanicum-infected hamsters, with the worm burden reduction of 87.2% and worm expulsion rate of 63.4%.
-
In VitroPyrantel pamoate (10 nM-10 μM; 72 h) shows good anti-A. suum and (0-168.2 M; 72 h) anti-N. americanus activity. Cell Viability Assay Cell Line:A. suum Concentration:10 nM-10 μM Incubation Time:72 h Result:Inhibited A. suum with a pEC50 value of 7.24.Cell Viability Assay Cell Line:N. americanus Concentration:0-168.2 M (0-100 μg/mL)Incubation Time:72 h Result:Inhibited third-stage larvae and adult of N. americanus with IC50 values of 2.0 and 7.6 mg/mL, respectively.
-
In VivoPyrantel pamoate (10 mg/kg; p.o.; single) reduces the worms in A. ceylanicum-infected hamsters, with the worm burden reduction of 87.2% and worm expulsion rate of 63.4%. Animal Model:Male Syrian Golden hamsters (3-week-old; A. ceylanicum-infected).Dosage:10 mg/kg Administration:Oral administration; single.Result:Exhibited worm burden reduction and worm expulsion rates of 87.2% and 63.4%, respectively.
-
SynonymsPyrantel pamoate
-
PathwayOthers
-
TargetOther Targets
-
RecptorAntiparasitic
-
Research AreaInfection
-
Indication——
Chemical Information
-
CAS Number22204-24-6
-
Formula Weight594.69
-
Molecular FormulaC34H30N2O6S
-
Purity>98% (HPLC)
-
SolubilityDMSO: 10 mM
-
SMILESCN1CCCN=C1/C=C/c2cccs2.c1ccc2c(c1)cc(c(c2Cc3c4ccccc4cc(c3O)C(=O)O)O)C(=O)O
-
Chemical Name(E)-1,4,5,6-Tetrahydro-1-methyl-2-(2-(2-thienyl)vinyl)pyrimidine 4,4'-methylenebis(3-hydroxy-2-naphthoate) (1:1)
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Salman AB. J Pediatr Surg. 1997 Apr;32(4): 585-7.
molnova catalog



related products
-
Malabaricone A
Malabaricone A is a bioactive chemical.
-
Lobaplatin (D-19466)
Lobaplatin (D-19466) is a diastereometric mixture of platinum(II) complexes. Lobaplatin (D-19466) shows activity for a variety of tumour types and is a promising antitumour chemotherapeutic agent.
-
ZY-444
ZY-444 is a small molecule inhibiting the progression of breast cancer by targeting pyruvate carboxylase.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


